1. Convert 0°F, 32°F, 70°F, and 212°F to Kelvin
0°F – 255.37 Kelvin
32°F – 273.15 Kelvin
70°F – 294.26 Kelvin
212°F – 373.15 Kelvin
2. Complete the Teaching Idea: States of Matter Simulation Lab by Kelly Vaughan. Complete the lab worksheet as if you were a student, and then post this on your blog. You can scan it or just take a picture of it.
3. In the States of Matter simulation, choose the Solid, Liquid, and Gas Tab at the top of the screen. Choose the water molecule and cool the water to 0 K. Describe how the water molecules are aligned and attracted to each other. Which atoms are attracted to which other atoms?
As you can see in the above picture, the white circles (hydrogen) are attracted to the orange circles (oxygen). You can also observe that when the molecules get together, they leave a circular shape in the middle of about 5 or 6 molecules.
4. Switch to the Phase Changes Tab on the States of Matter simulation. Notice how on the bottom right there is a small red dot that indicates where the system is at as far as temperature, pressure and state of matter. Play with the simulation to notice changes, notice that when you push down the pressure can go way up and explode the box. On your blog, report a temperature and pressure required to make oxygen a liquid. This is sometimes how the oxygen exists in pressurized oxygen tanks, perhaps like ones you may use to go diving.
Temperature - 126 K
Pressure - 8 ATM
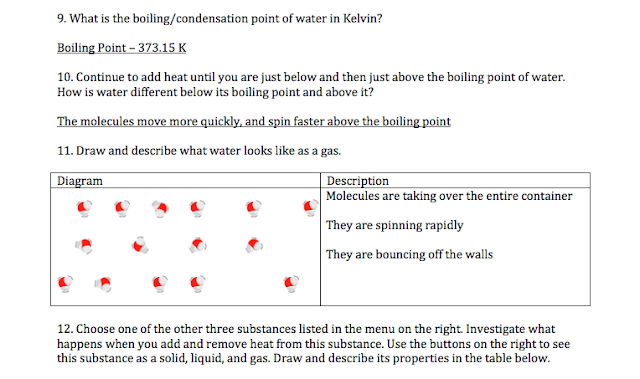
5. List and describe at least two Science Standards that this activity addresses.
D.4.3 Understand that substances can exist in different states - solid, liquid, gas.
This standard is clearly addressed in this activity. We were asked to look at the differences between solids, liquids, and gasses - therefore it is obvious that the above standard was addressed.
D.4.4 Observe and describe changes in form, temperature, color, speed, and direction of objects and construct explanations for the changes.
This standard was addressed multiple times throughout this activity. I observed the form changes of water and argon, I analyzed the different speeds of molecules at different temperatures, I adjusted the temperature in question four, and I constructed explanations.








No comments:
Post a Comment